Ready to learn more about making NeuroStar® a part of your practice? Already a NeuroStar customer and have a question for us? Whatever you need, we’re here to help.
TREATMENT
When Drugs Don’t Work, Look to NeuroStar®

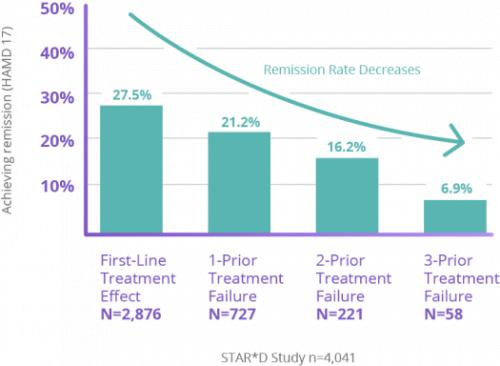
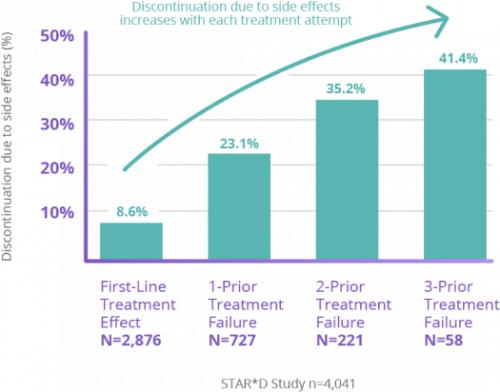
STAR*D Study: The Limitations of Antidepressants
Patients Are Searching for a New Standard of Care
Perhaps most compelling, a recent study of 500 depression patients showed that 67% of patients with MDD aren’t happy with their current treatment and quality of life, and are actively searching for new treatment solutions.10,14,26
For patients who haven’t found relief from antidepressants, trying another medication may not be the answer.

The Best Option When Medications Fail
NeuroStar has achieved 83% response and 62% remission rates for patients with MDD, according to results from a retrospective study of real-world outcomes in nearly 8,000 patients and over 100 sites.5 The NeuroStar® Advanced Therapy Outcomes Registry, which is the largest data set in MDD, is comprised of more than 15,000 patients across 118 NeuroStar practice sites.7,8
NeuroStar performance is backed by the world’s largest depression Outcomes Registry**, with evaluable data on over 15,000 patients since its initiation in 2016. This real-world data was collected from 118 NeuroStar practice sites.2,6
An Advanced Solution for Patients with MDD

Over 6.1 million treatments delivered. Over 16,500 people on our patient registry. Over 169,000 lives transformed.
There’s a reason NeuroStar is the market leader in TMS therapy for depression.
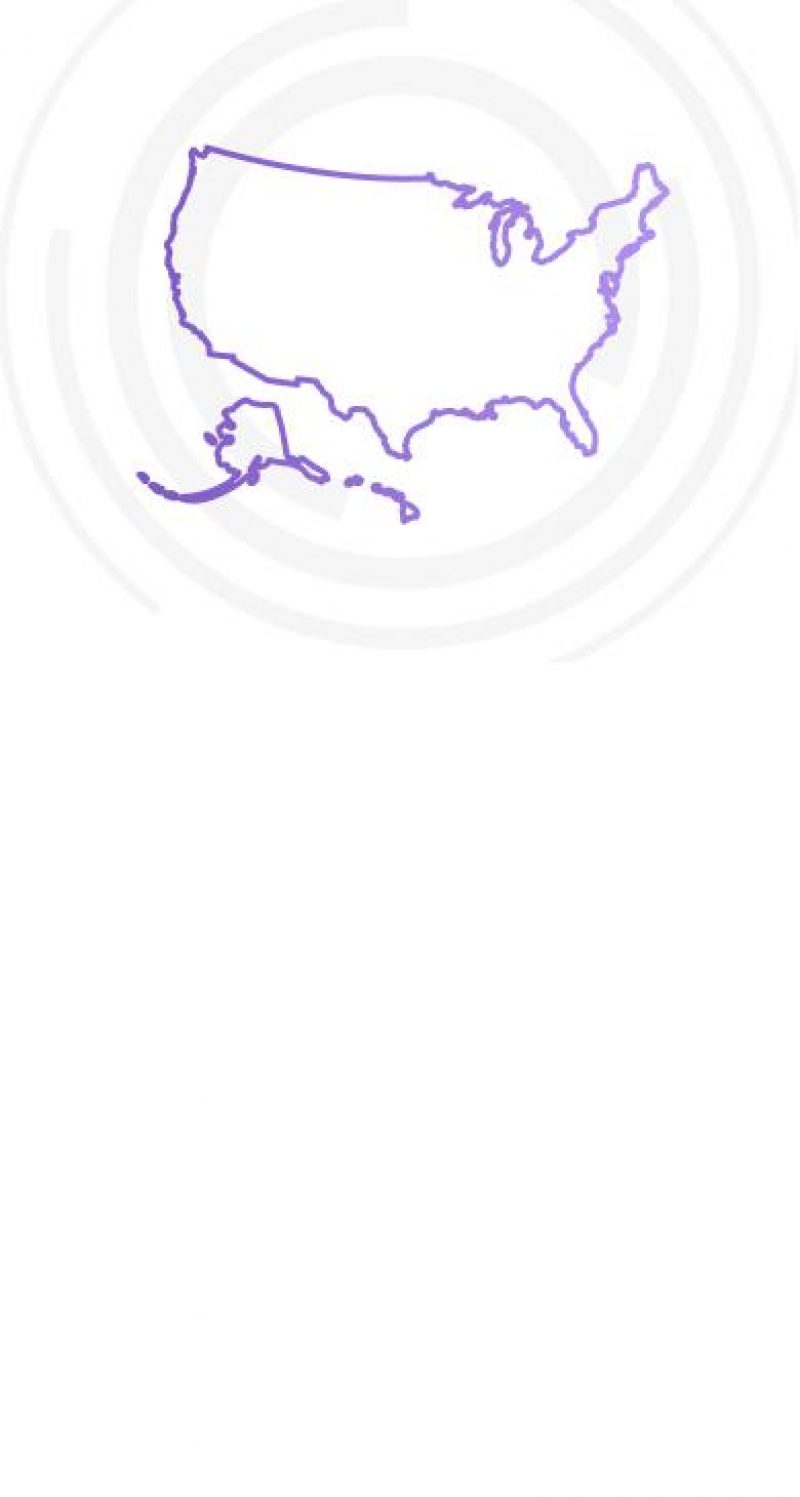
Insurance Coverage in All 50 States
NeuroStar is widely reimbursed, with insurance coverage in all 50 states, including Medicare and Tricare. Over 95 major U.S. private insurers provide coverage for NeuroStar, representing 300 million people. Medicare provides 100% coverage for NeuroStar (61 million covered people).
NeuroStar has a specialized reimbursement team with the expertise to support you throughout our partnership.
Fill in the Blanks for Your Practice
Provide your contact info and we’ll put you in touch with a NeuroStar representative.