A Better Option for Those with Depression
Neuronetics cares about practice success and we care about getting people better. We’re committed to improving quality of life for people suffering from psychiatric disorders. We do this by taking a patient-centric approach to developing medical devices that provide non-drug, non-invasive treatments.
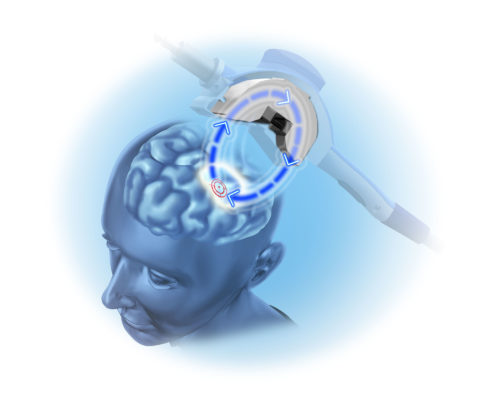
Neuronetics’ NeuroStar® Advanced Therapy for Mental Health is today’s leading TMS (transcranial magnetic stimulation) treatment and is backed by more clinical evidence than any other TMS technology for depression.1 The largest TMS company in the industry, NeuroStar helps our practices succeed by providing exceptional ongoing support.
Looking forward, Neuronetics is working to expand our capabilities to cover additional indications.
BROAD REIMBURSEMENT
More than 300 million people have insurance plans that cover NeuroStar.
CLINICAL PROOF
The largest clinical data set of any TMS therapy for depression and the world’s largest depression Outcomes Registry.1
SALES & CUSTOMER SUPPORT
The largest direct sales and customer support teams in the industry.
“The work we are continuing to do each and every day to help others fight and overcome depression really is life-changing.”
A Unique Approach to Neurohealth
The first line of treatment for depression is usually antidepressant medications, which are not always successful and may cause unpleasant side effects. NeuroStar offers an FDA-cleared treatment approach that has been proven safe, proven effective, and proven durable over 12 months.3,16,19
Putting Customers & Patients First
The Neuronetics leadership team is made up of a group of dedicated and experienced individuals who understand the needs of both NeuroStar practices and the patients they serve. We demand the highest quality from ourselves. We inspire transformation and positive change in each other and the world around us. We succeed through collaboration.
The Opportunity to Transform Lives
Neuronetics is a company inspired by the opportunity to transform lives through compassion, innovation, and growth. For more information on what we do, how to join our team, or ways you can invest in our future, contact us today.
Contact Neuronetics

